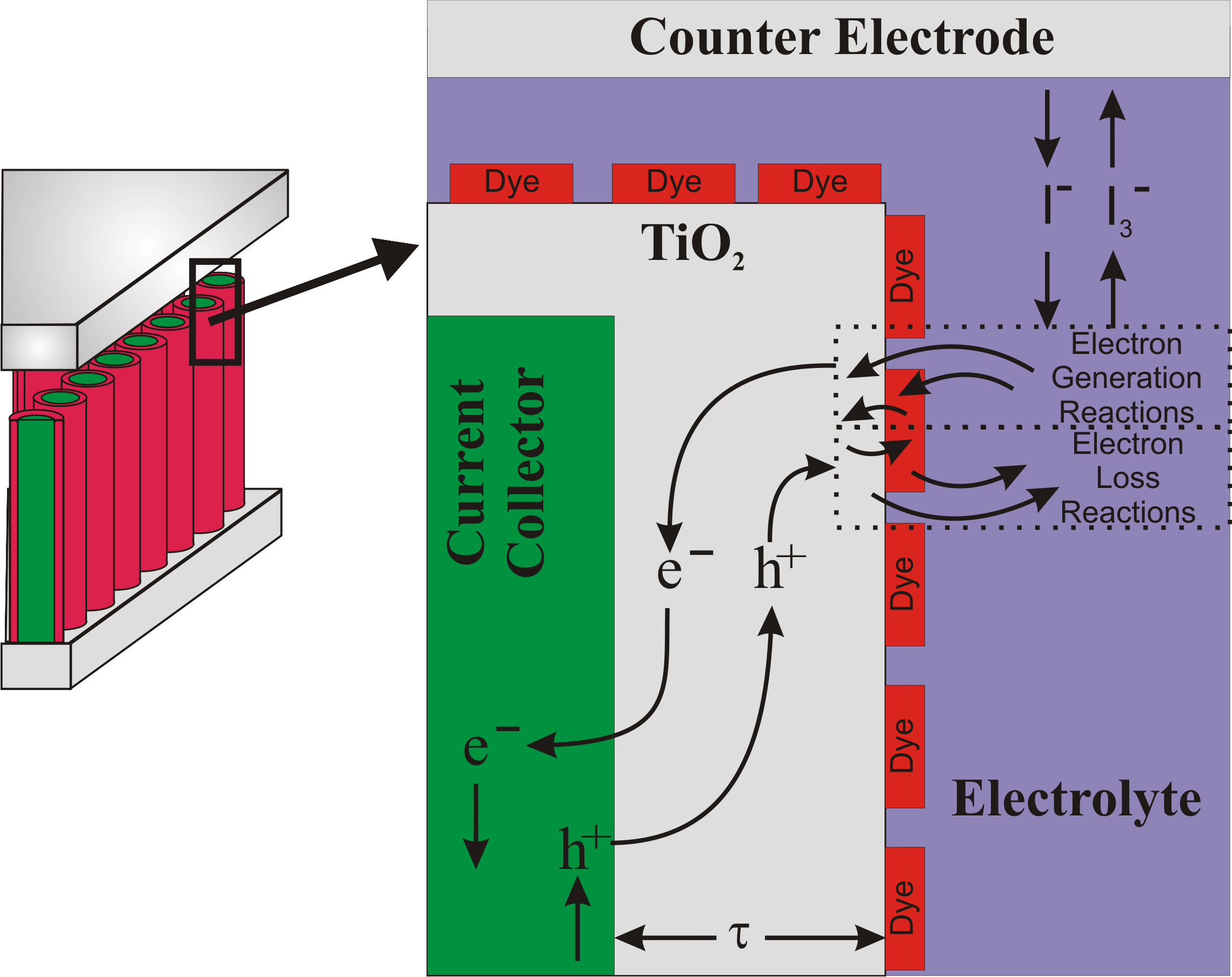
 Dye-sensitized solar cells (DSSCs) mimic photosynthesis. A chromophore sensitizer (usually a ruthenium-based dye) is used in DSSCs to harvest photons. Photons absorbed by a monolayer of the dye create excitons that are rapidly split resulting in the injection of electrons into the conduction band of a large band gap semiconductor, typically titania. The electron collecting layer in a DSSC is a photoanode (approximately 10 microns thick) of interconnected nanometer-sized titania particles. The large surface area associated with these films provides significant light absorption and, subsequently, the number of electrons injected into titania. The hole from the exciton is injected into an electrolyte, typically containing the iodide/triiodide couple as a redox system. The redox system is regenerated by the electrons at the counter electrode.
Dye-sensitized solar cells (DSSCs) mimic photosynthesis. A chromophore sensitizer (usually a ruthenium-based dye) is used in DSSCs to harvest photons. Photons absorbed by a monolayer of the dye create excitons that are rapidly split resulting in the injection of electrons into the conduction band of a large band gap semiconductor, typically titania. The electron collecting layer in a DSSC is a photoanode (approximately 10 microns thick) of interconnected nanometer-sized titania particles. The large surface area associated with these films provides significant light absorption and, subsequently, the number of electrons injected into titania. The hole from the exciton is injected into an electrolyte, typically containing the iodide/triiodide couple as a redox system. The redox system is regenerated by the electrons at the counter electrode.
DSSCs have achieved conversion efficiencies as high as ~11%. However, further improvement to the efficiencies of DSSCs are limited by the competition between electron injection and the loss mechanisms from interfacial charge recombination and back reactions. Without a localized field driving electrons to the current collector, interfacial electrons can recombine with the photoactive dye or back-react with the electrolyte. This effect places an upper limit on the film thickness of the photoanode and the amount of absorbed light.
Since the thickness of the titania photoanode cannot be increased, dye uptake may be important in determining DSSC conversion efficiencies. Dye insertion through wet chemical or gas phase approaches are subject to capillary forces and condensation leading to inefficient coating of the titania nanostructures. Supercritical fluids (SCFs) cannot be condensed to a liquid phase minimizing these problems. We are investigating whether SCF impregnation can improve DSSC performance by providing better impregnation of the dye. Improved dye adsorption on the titania nanoparticles may retard interfacial charge recombination. A reduction in interfacial charge recombination would also allow thicker films to be used, providing additional increases in performance.
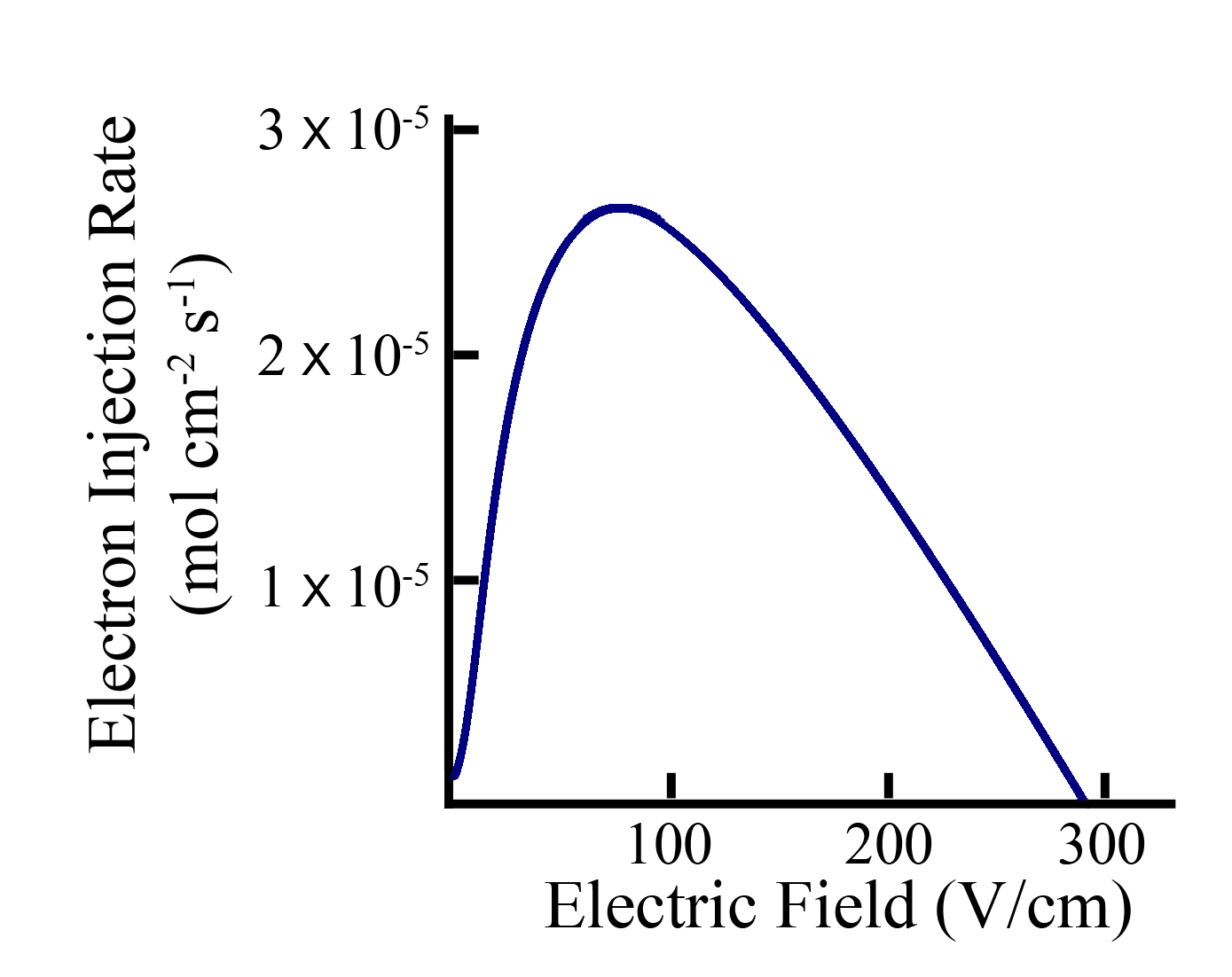 The development of new photoanodes may also provide significant improvement to DSSC performance. Nanowire arrays are promising architectures that have improved electron transport and charge injection due to an electric field. This electric field helps selectively drive the injected electrons from the surrounding electrolyte, achieving transport dynamics two orders of magnitude faster than nanoparticle-based DSSCs. While the benefits of nanowire-based photoanodes are exciting, devices fabricated thus far have not been able to take full advantage of the benefits that nanowires offer to electron transport, short circuit current, and open circuit voltage. We are conducting a combination of experimental and computational studies to understand the electron transport within nanowire-based DSSCs. The aim is to fabricate high-density arrays of nanowires with surface area comparable to the nanoparticle-based DSSCs.
The development of new photoanodes may also provide significant improvement to DSSC performance. Nanowire arrays are promising architectures that have improved electron transport and charge injection due to an electric field. This electric field helps selectively drive the injected electrons from the surrounding electrolyte, achieving transport dynamics two orders of magnitude faster than nanoparticle-based DSSCs. While the benefits of nanowire-based photoanodes are exciting, devices fabricated thus far have not been able to take full advantage of the benefits that nanowires offer to electron transport, short circuit current, and open circuit voltage. We are conducting a combination of experimental and computational studies to understand the electron transport within nanowire-based DSSCs. The aim is to fabricate high-density arrays of nanowires with surface area comparable to the nanoparticle-based DSSCs.
Related Publications
J.J. Hill, N. Banks, K. Haller, M.E. Orazem, and K.J. Ziegler. An interfacial and bulk charge transport model for dye-sensitized solar cells based on photoanodes consisting of core-shell nanowire arrays. J. Am. Chem. Soc., 2011, 133, 18663. [link]
F. Rajab, D. Loaring, and K.J. Ziegler. Preparing thick, defect-free films of anatase titania for dye-sensitized solar cells. Thin Solid FIlms, 2011, 519, 6598. [link]
J.J. Hill, K. Haller, B. Gelfand, and K.J. Ziegler. Eliminating capillary coalescence of nanowire arrays with applied electric fields. ACS Appl. Mater. Inter. 2010, 2, 1992. [link]
K.J. Ziegler, B. Polyakov, J.S. Kulkarni, T.A. Crowley, K.M. Ryan, M.A. Morris, D. Erts, and J.D. Holmes. Conductive films of ordered nanowire arrays. J. Mater. Chem. 2004, 14, 585. [link]
 Dye-sensitized Solar Cells
Dye-sensitized Solar Cells  Dye-sensitized solar cells (DSSCs) mimic photosynthesis. A chromophore sensitizer (usually a ruthenium-based dye) is used in DSSCs to harvest photons. Photons absorbed by a monolayer of the dye create excitons that are rapidly split resulting in the injection of electrons into the conduction band of a large band gap semiconductor, typically titania. The electron collecting layer in a DSSC is a photoanode (approximately 10 microns thick) of interconnected nanometer-sized titania particles. The large surface area associated with these films provides significant light absorption and, subsequently, the number of electrons injected into titania. The hole from the exciton is injected into an electrolyte, typically containing the iodide/triiodide couple as a redox system. The redox system is regenerated by the electrons at the counter electrode.
Dye-sensitized solar cells (DSSCs) mimic photosynthesis. A chromophore sensitizer (usually a ruthenium-based dye) is used in DSSCs to harvest photons. Photons absorbed by a monolayer of the dye create excitons that are rapidly split resulting in the injection of electrons into the conduction band of a large band gap semiconductor, typically titania. The electron collecting layer in a DSSC is a photoanode (approximately 10 microns thick) of interconnected nanometer-sized titania particles. The large surface area associated with these films provides significant light absorption and, subsequently, the number of electrons injected into titania. The hole from the exciton is injected into an electrolyte, typically containing the iodide/triiodide couple as a redox system. The redox system is regenerated by the electrons at the counter electrode.  The development of new photoanodes may also provide significant improvement to DSSC performance.
The development of new photoanodes may also provide significant improvement to DSSC performance. 