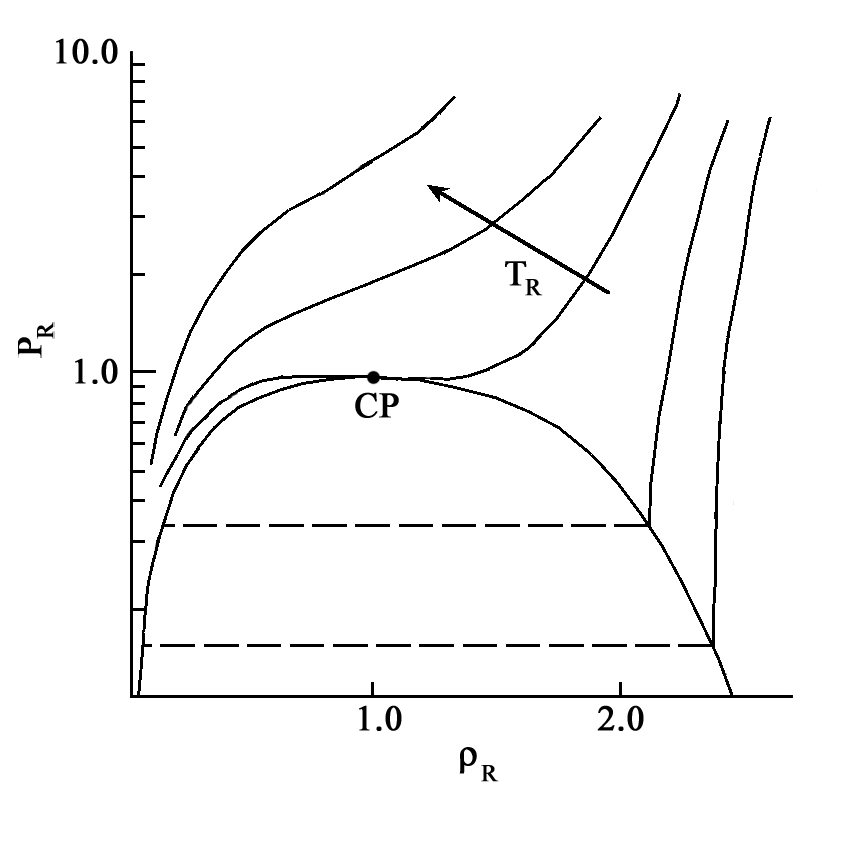
 A supercritical fluid (SCF) is a compound, mixture or element above its critical pressure and critical temperature but below the pressure required to condense it to a solid. Many of the physical properties of SCFs vary with density. Small changes in pressure or temperature can cause large variations in the density of a SCF between gas-like and liquid-like values. Hence conducting reactions in SCFs affords opportunities to manipulate the reaction environment, e.g. viscosity, diffusivity and surface tension, through the control of pressure and temperature to enhance solubilities of reactants and products and to eliminate transport restrictions on reaction rates. The search for environmentally benign solvents has raised the interest in SCFs as replacements for hazardous organic solvents. SCFs have a wide variety of applications including food processing, pharmaceuticals, polymers, petrochemical separations, petroleum refining, treatment of contaminated soils, waste destruction, cleaning of electronics, drying, sprays, materials formation such as particles and fibers, and many others. The two most frequently used SCFs are supercritical CO2 (sc-CO2) and supercritical water (SCW) due to their abundancy and relative non-flammability and non-toxicity.
A supercritical fluid (SCF) is a compound, mixture or element above its critical pressure and critical temperature but below the pressure required to condense it to a solid. Many of the physical properties of SCFs vary with density. Small changes in pressure or temperature can cause large variations in the density of a SCF between gas-like and liquid-like values. Hence conducting reactions in SCFs affords opportunities to manipulate the reaction environment, e.g. viscosity, diffusivity and surface tension, through the control of pressure and temperature to enhance solubilities of reactants and products and to eliminate transport restrictions on reaction rates. The search for environmentally benign solvents has raised the interest in SCFs as replacements for hazardous organic solvents. SCFs have a wide variety of applications including food processing, pharmaceuticals, polymers, petrochemical separations, petroleum refining, treatment of contaminated soils, waste destruction, cleaning of electronics, drying, sprays, materials formation such as particles and fibers, and many others. The two most frequently used SCFs are supercritical CO2 (sc-CO2) and supercritical water (SCW) due to their abundancy and relative non-flammability and non-toxicity.
Since SCFs offer great chemical flexibility and synthetic tunability, they have recently been utilised in the synthesis of nanocrystalline materials. The ability to control the mass-transfer properties of a SCF is particularly important for the nucleation and growth of crystals or films. Combining the benefits of high temperatures used in aerosol methods and the highly successful use of wet chemical arrested-growth procedures for producing nanoparticles, silicon and germanium nanocrystals have been synthesized in sc-hexane. SCFs have also been instrumental in fabricating metal, metal oxide, and semiconductor nanowires within mesoporous materials. The high-diffusivity, high precursor solubility, and reduced surface tension of the SCF results in rapid nucleation and growth of the nanowires within the pores reducing the reaction time for pore inclusion by at least an order of magnitude compared to chemical vapour deposition (CVD). Additionally, SCFs cannot be condensed to a liquid phase reducing the problems of pore plugging and incomplete inclusion seen with CVD, electrodeposition, and incipient wetness techniques.
Related Publications
J.P. Hanrahan, M.P. Copley, K.J. Ziegler , T.R. Spalding, M.A. Morris, D.C. Steytler, R.K. Heenan, R. Schweins, and J.D. Holmes. Pore size engineering in mesoporous silicas using supercritical CO2. Langmuir. 2005, 21,4163. [link]
J.D. Holmes, D.M. Lyons, and K.J. Ziegler. Supercritical fluid synthesis of metal and semiconductor nanowires. Chem. Eur. J. 2003, 9, 2144. [link]
J.D. Holmes, K.J. Ziegler, M. Audriani, C.T. Lee, Jr., P.A. Bhargava, D.C. Steytler, and K.P. Johnston. Buffering the aqueous phase pH in water-in-CO2 microemulsions. J. Phys. Chem. B. 1999, 103, 5703. [link]
J. Chlistunoff, K.J. Ziegler, L. Lasdon, and K.P. Johnston. Nitric/nitrous acid equilibria in supercritical water. J. Phys. Chem. A. 1999, 103, 1678. [link]
J.P. Hanrahan, K.J. Ziegler, J.D. Glennon, D.C. Steytler, J. Eastoe, A. Dupont, and J.D. Holmes. pH switching for the selective extraction of metal ions into supercritical CO2. Langmuir. 2003, 19, 3145. [link]
 Supercritical Fluids
Supercritical Fluids  A supercritical fluid (SCF) is a compound, mixture or element above its critical pressure and critical temperature but below the pressure required to condense it to a solid. Many of the physical properties of SCFs vary with density. Small changes in pressure or temperature can cause large variations in the density of a SCF between gas-like and liquid-like values. Hence conducting reactions in SCFs affords opportunities to manipulate the reaction environment, e.g. viscosity, diffusivity and surface tension, through the control of pressure and temperature to enhance solubilities of reactants and products and to eliminate transport restrictions on reaction rates. The search for environmentally benign solvents has raised the interest in SCFs as replacements for hazardous organic solvents. SCFs have a wide variety of applications including food processing, pharmaceuticals, polymers, petrochemical separations, petroleum refining, treatment of contaminated soils, waste destruction, cleaning of electronics, drying, sprays, materials formation such as particles and fibers, and many others. The two most frequently used SCFs are supercritical CO2 (sc-CO2) and supercritical water (SCW) due to their abundancy and relative non-flammability and non-toxicity.
A supercritical fluid (SCF) is a compound, mixture or element above its critical pressure and critical temperature but below the pressure required to condense it to a solid. Many of the physical properties of SCFs vary with density. Small changes in pressure or temperature can cause large variations in the density of a SCF between gas-like and liquid-like values. Hence conducting reactions in SCFs affords opportunities to manipulate the reaction environment, e.g. viscosity, diffusivity and surface tension, through the control of pressure and temperature to enhance solubilities of reactants and products and to eliminate transport restrictions on reaction rates. The search for environmentally benign solvents has raised the interest in SCFs as replacements for hazardous organic solvents. SCFs have a wide variety of applications including food processing, pharmaceuticals, polymers, petrochemical separations, petroleum refining, treatment of contaminated soils, waste destruction, cleaning of electronics, drying, sprays, materials formation such as particles and fibers, and many others. The two most frequently used SCFs are supercritical CO2 (sc-CO2) and supercritical water (SCW) due to their abundancy and relative non-flammability and non-toxicity. 