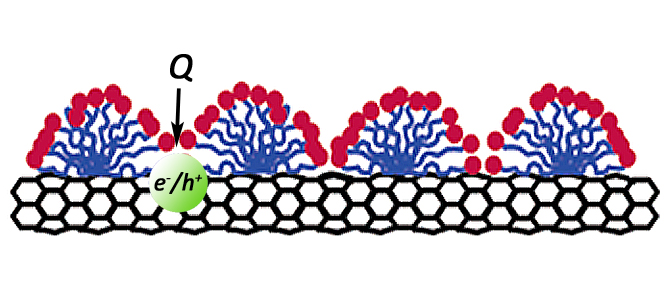
 Since the discovery of single wall carbon nanotubes (SWCNTs) they have attracted much attention due to their unique electronic and mechanical properties. Since all atoms in a SWCNT exist on the surface, their properties depend on the interaction with other materials. For this reason, SWCNTs have excellent sensing capabilities. Altering the interface around SWCNTs has also been instrumental to the significant advances made in dispersing and separating nanotubes. For example, coating the SWCNT with surfactants has enabled characterization and (n,m) type separation. The SWCNT interface is also important in determining the properties of devices, electrodes, and composite structures. However, little is known about the structure of surfactants around nanotubes or the affect of the environment on SWCNT characterization. The limited understanding of environmental effects to SWCNT properties is partially due to the unknown or poorly characterized interfaces. Three models of surfactant assembly on SWCNT sidewalls have been suggested: cylindrical, hemimicellar, and random assemblies. The surfactant structure has important implications on the PL emission intensity. For example, nonuniform structures and exposed sidewalls have effects on PL emission because of the quenching effects of protons or water. Therefore, the PL emission intensity of SWCNTs may depend on both the structure of the interface and the environment surrounding SWCNTs.
Since the discovery of single wall carbon nanotubes (SWCNTs) they have attracted much attention due to their unique electronic and mechanical properties. Since all atoms in a SWCNT exist on the surface, their properties depend on the interaction with other materials. For this reason, SWCNTs have excellent sensing capabilities. Altering the interface around SWCNTs has also been instrumental to the significant advances made in dispersing and separating nanotubes. For example, coating the SWCNT with surfactants has enabled characterization and (n,m) type separation. The SWCNT interface is also important in determining the properties of devices, electrodes, and composite structures. However, little is known about the structure of surfactants around nanotubes or the affect of the environment on SWCNT characterization. The limited understanding of environmental effects to SWCNT properties is partially due to the unknown or poorly characterized interfaces. Three models of surfactant assembly on SWCNT sidewalls have been suggested: cylindrical, hemimicellar, and random assemblies. The surfactant structure has important implications on the PL emission intensity. For example, nonuniform structures and exposed sidewalls have effects on PL emission because of the quenching effects of protons or water. Therefore, the PL emission intensity of SWCNTs may depend on both the structure of the interface and the environment surrounding SWCNTs.
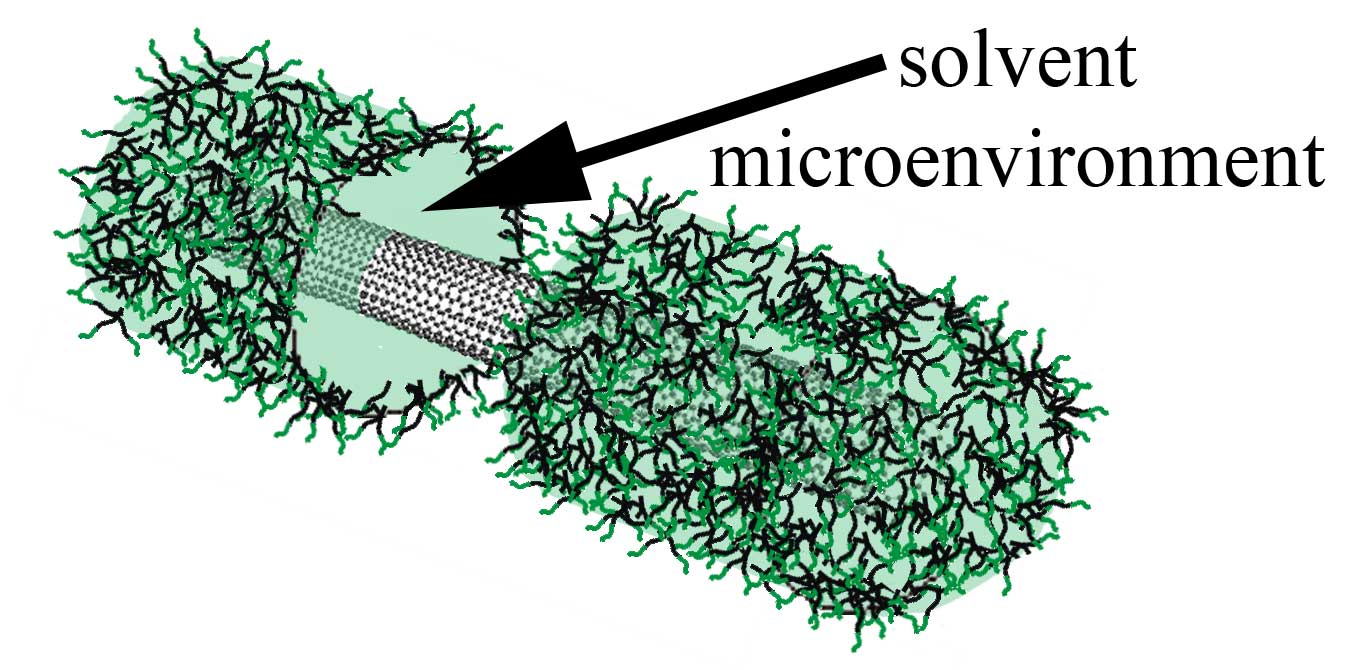 We are working to understand the role of nanotube interfaces and develop new methods to control the surface properties of SWCNTs. A significant portion of our work in this area uses immiscible liquid systems. Mixing an aqueous SWCNT suspension with an immicsible organic solvent results in solvatochromic shifts of the spectra. We have shown that these organic solvents swell the hydrophobic core of surfactant micelles surrounding SWNTs, yielding an emulsion-like microenvironment surrounding the SWCNTs. These microenvironments have already provided opportunities to prepare new coatings and to study the photophysics of SWCNTs in different microenvironments. This work has shown that the peak position (Eii) for different environments is related to the Onsager polarity function.
We are working to understand the role of nanotube interfaces and develop new methods to control the surface properties of SWCNTs. A significant portion of our work in this area uses immiscible liquid systems. Mixing an aqueous SWCNT suspension with an immicsible organic solvent results in solvatochromic shifts of the spectra. We have shown that these organic solvents swell the hydrophobic core of surfactant micelles surrounding SWNTs, yielding an emulsion-like microenvironment surrounding the SWCNTs. These microenvironments have already provided opportunities to prepare new coatings and to study the photophysics of SWCNTs in different microenvironments. This work has shown that the peak position (Eii) for different environments is related to the Onsager polarity function.
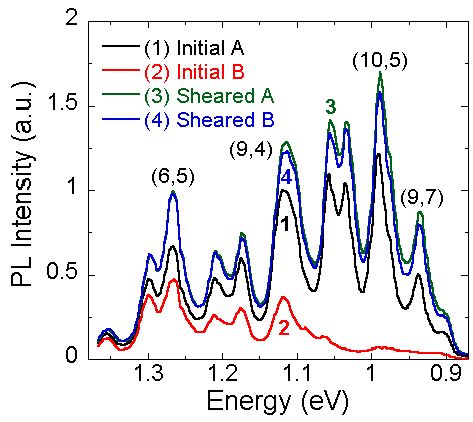 Interestingly, the surfactant in SDS-SWCNT suspensions can rearrange to more stable structures around the nanotube after the solvent evaporates from the microenvironments. We reported a similar 'annealing' phenomenon with mechanical shearing. The rearrangement of the surfactant layer has a dramatic effect on the PL intensity of the suspension. These changes are associated with a reduction in the surfactant permeability to species that quench the PL, as decribed above. These PL increases persist for months and demonstrate improved dispersion characteristics and quantum yields (~1%). Differences in the surfactant structure may also help explain discrepancies in the PL intensity of different suspensions or between research groups. The initial suspensions A and B (curves 1 and 2) exhibit different PL intensity for the largest diameter SWCNTs (lowest energy). However, the PL intensity is nearly identical after shearing both suspensions.
Interestingly, the surfactant in SDS-SWCNT suspensions can rearrange to more stable structures around the nanotube after the solvent evaporates from the microenvironments. We reported a similar 'annealing' phenomenon with mechanical shearing. The rearrangement of the surfactant layer has a dramatic effect on the PL intensity of the suspension. These changes are associated with a reduction in the surfactant permeability to species that quench the PL, as decribed above. These PL increases persist for months and demonstrate improved dispersion characteristics and quantum yields (~1%). Differences in the surfactant structure may also help explain discrepancies in the PL intensity of different suspensions or between research groups. The initial suspensions A and B (curves 1 and 2) exhibit different PL intensity for the largest diameter SWCNTs (lowest energy). However, the PL intensity is nearly identical after shearing both suspensions.
Related Publications
J.G. Clar, C.A. Silvera-Batista, S. Youn, J.-C. J. Bonzongo, and K.J. Ziegler. Interactive forces between SDS-suspended single-wall carbon nanotubes and agarose gels. J. Am. Chem. Soc., 2013, in press. [link]
C. Silvera-Batista and K.J. Ziegler. Swelling the Hydrophobic Core of Surfactant–Suspended Single Wall Carbon Nanotubes: A SANS Study. Langmuir, 2011, 27, 11372. [link]
C. Silvera-Batista, D. Scott, S. McLeod, and K.J. Ziegler. A mechanistic study of the selective retention of SDS-suspended single-wall carbon nanotubes on agarose gels. J. Phys. Chem. C, 2011, 115, 9361.
[
link
]
C. Silvera-Batista, R.K. Wang, P. Weinberg, and K.J. Ziegler. Solvatochromic shifts of single-walled carbon nanotubes in nonpolar microenvironments. Phys. Chem. Chem. Phys. 2010, 12, 6990. [link]
W.-C. Chen, R.K. Wang, and K.J. Ziegler. Coating individual single-walled carbon nanotubes with Nylon 6, 10 through emulsion polymerization. ACS Appl. Mater. Inter. 2009, 1, 1821. [link]
R.K. Wang, W.-C. Chen, D.K. Campos, and K.J. Ziegler. Swelling the micelle core surrounding single-walled carbon nanotubes with water-immiscible organic solvents. J. Am. Chem. Soc. 2008. 130, 16330. [link]
 Single Wall Carbon Nanotubes
Single Wall Carbon Nanotubes  Since the discovery of single wall carbon nanotubes (SWCNTs) they have attracted much attention due to their unique electronic and mechanical properties. Since all atoms in a SWCNT exist on the surface, their properties depend on the interaction with other materials. For this reason, SWCNTs have excellent sensing capabilities. Altering the interface around SWCNTs has also been instrumental to the significant advances made in dispersing and separating nanotubes. For example, coating the SWCNT with surfactants has enabled characterization and (n,m) type separation. The SWCNT interface is also important in determining the properties of devices, electrodes, and composite structures. However, little is known about the structure of surfactants around nanotubes or the affect of the environment on SWCNT characterization. The limited understanding of environmental effects to SWCNT properties is partially due to the unknown or poorly characterized interfaces. Three models of surfactant assembly on SWCNT sidewalls have been suggested: cylindrical, hemimicellar, and random assemblies. The surfactant structure has important implications on the PL emission intensity. For example, nonuniform structures and exposed sidewalls have effects on PL emission because of the quenching effects of protons or water. Therefore, the PL emission intensity of SWCNTs may depend on both the structure of the interface and the environment surrounding SWCNTs.
Since the discovery of single wall carbon nanotubes (SWCNTs) they have attracted much attention due to their unique electronic and mechanical properties. Since all atoms in a SWCNT exist on the surface, their properties depend on the interaction with other materials. For this reason, SWCNTs have excellent sensing capabilities. Altering the interface around SWCNTs has also been instrumental to the significant advances made in dispersing and separating nanotubes. For example, coating the SWCNT with surfactants has enabled characterization and (n,m) type separation. The SWCNT interface is also important in determining the properties of devices, electrodes, and composite structures. However, little is known about the structure of surfactants around nanotubes or the affect of the environment on SWCNT characterization. The limited understanding of environmental effects to SWCNT properties is partially due to the unknown or poorly characterized interfaces. Three models of surfactant assembly on SWCNT sidewalls have been suggested: cylindrical, hemimicellar, and random assemblies. The surfactant structure has important implications on the PL emission intensity. For example, nonuniform structures and exposed sidewalls have effects on PL emission because of the quenching effects of protons or water. Therefore, the PL emission intensity of SWCNTs may depend on both the structure of the interface and the environment surrounding SWCNTs.  We are working to understand the role of nanotube interfaces and develop new methods to control the surface properties of SWCNTs. A significant portion of our work in this area uses immiscible liquid systems. Mixing an aqueous SWCNT suspension with an immicsible organic solvent results in solvatochromic shifts of the spectra. We have shown that these organic solvents swell the hydrophobic core of surfactant micelles surrounding SWNTs, yielding an emulsion-like microenvironment surrounding the SWCNTs. These microenvironments have already provided opportunities to prepare
We are working to understand the role of nanotube interfaces and develop new methods to control the surface properties of SWCNTs. A significant portion of our work in this area uses immiscible liquid systems. Mixing an aqueous SWCNT suspension with an immicsible organic solvent results in solvatochromic shifts of the spectra. We have shown that these organic solvents swell the hydrophobic core of surfactant micelles surrounding SWNTs, yielding an emulsion-like microenvironment surrounding the SWCNTs. These microenvironments have already provided opportunities to prepare  Interestingly, the surfactant in SDS-SWCNT suspensions can rearrange to more stable structures around the nanotube after the solvent evaporates from the microenvironments. We reported a similar 'annealing' phenomenon with mechanical shearing. The rearrangement of the surfactant layer has a dramatic effect on the PL intensity of the suspension. These changes are associated with a reduction in the surfactant permeability to species that quench the PL, as decribed above. These PL increases persist for months and demonstrate improved dispersion characteristics and quantum yields (~1%). Differences in the surfactant structure may also help explain discrepancies in the PL intensity of different suspensions or between research groups. The initial suspensions A and B (curves 1 and 2) exhibit different PL intensity for the largest diameter SWCNTs (lowest energy). However, the PL intensity is nearly identical after shearing both suspensions.
Interestingly, the surfactant in SDS-SWCNT suspensions can rearrange to more stable structures around the nanotube after the solvent evaporates from the microenvironments. We reported a similar 'annealing' phenomenon with mechanical shearing. The rearrangement of the surfactant layer has a dramatic effect on the PL intensity of the suspension. These changes are associated with a reduction in the surfactant permeability to species that quench the PL, as decribed above. These PL increases persist for months and demonstrate improved dispersion characteristics and quantum yields (~1%). Differences in the surfactant structure may also help explain discrepancies in the PL intensity of different suspensions or between research groups. The initial suspensions A and B (curves 1 and 2) exhibit different PL intensity for the largest diameter SWCNTs (lowest energy). However, the PL intensity is nearly identical after shearing both suspensions. 